Different methods of expressing the concentration of solution

In Chemistry,
“Concentration” expresses the amount of solute present in the solution. Suppose
you have been given two jars containing lemon juice and added different
quantities of sugar. How will you express its concentration? You will say lemon
juice in one jar is “too sweet” and “less sweet” in another. But Chemistry
doesn’t use the word “too” to express concentrations. We have to define it.
A solution in which a
relative amount of solute is less is known as a dilute solution, and one with a
large amount of solute is known as a concentrated solution. But these are the
relative terms and do not give quantitative information about the solution. Thus,
in this article, we will look at the different methods of expressing the
concentration of solutions.
So, let’s start!
1) Solution
concentration or strength:
It describes the amount of solute present in one litre of solution. It is denoted by C or S, and its unit is g/L. It is also known as the strength of the solution.
For example: If 5 grams of salt is added to 0.5 L water, then the concentration of the solution will be 5/0.5 = 10 g/L.
2) Concentration
in parts per million (ppm):
It describes the part of a particular component per million (106) parts of the solution. It is expressed as milligrams of solute present in one litre of solution, i.e., mg/L. Another unit for ppm is μg/mL.
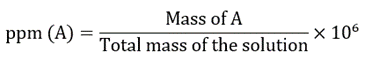
There
are some more units used to express concentration in parts: parts per billion
(ppb, parts per 109) and parts per trillion (ppt, parts per 1012)
and parts per quadrillion (ppq, parts per 1015).
3) Mass/weight
percentage or Weight/mass percentage (w/w):
The mass percentage of a solution is the concentration of the solution expressed as the per cent of one component in the solution by mass. Suppose a solution consists of two components: solute A and solvent B, then the mass percentage of A will be

For
example, 30% (w/w) glucose means 30 grams of glucose (solute) is present in 100
grams of the solution, i.e., 30 grams of glucose is dissolved in 70 grams of
water.
4) Volume
percentage (v/v):
The volume percentage of a solution is the concentration of the solution expressed as the per cent of one component in the solution by volume. Suppose a solution consists of two components: solute A and solvent B, then the volume percentage of A will be
For
example, 20% (v/v) ethanol means 20 mL of ethanol is present in 100 mL of the
solution, i.e., 20 grams of ethanol is mixed with 80 mL of water.
5) Mass
by Volume percentage (w/v):
It
means the mass of a solute dissolved per 100 mL of the solution. This
phenomenon is majorly used in the pharmaceutical industry.
6) Molarity
(M):
Molarity is known as the number of moles of solute present in one litre of the solution. It is denoted by ‘M’, and its unit is ‘Molar’ or ‘mol/L’. A mole is the amount of the substance that contains 6.022 X1023 entities, like particles, atoms, ions, molecules, etc., of the given substance. Molarity depends on the temperature.


Suppose
a solution of sucrose is marked as 0.5 M. This means that 0.5 moles of sucrose are
dissolved in one litre of the solution.
7) Molality
(m):
The number of moles of solute dissolved in one kg of the solvent is known as its Molality. It is denoted by ‘m’, and its unit is ‘molal’ or ‘mol/kg’. It is independent of temperature.

Suppose a NaCl solution is marked as 0.25
m; this means that 0.25 moles of NaCl are dissolved in one kg of the solvent.
8) Normality
(N):
Normality is the number of gram equivalents of solute dissolved in one litre of the solution. Normality is denoted by N, and its unit is ‘Normal’ or ‘equivalents/L’.
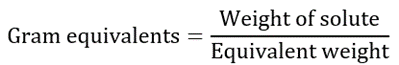
9) Mole
fraction:
Mole fraction is the ratio of moles of one component to the total moles of all the components present in the solution. Suppose we have a solution containing solute A and solvent B. Let nA and nB be solute A and solvent B moles, respectively. So, the mole fractions, xA and xB, of A and B, respectively, can be written as


10) Formality:
The
number of gram formula units present per litre of solution is known as a formality
and is denoted by F. When formula mass equals the molecular mass, formality equals
the molarity. Formality is useful for ionic compounds in which the molecule
does not exist.
Conclusion:
In stoichiometric calculations,
determining the number of moles and concentrations is important. For preparing
solutions during practicals, it is necessary to calculate the weight of the
solute that must be weighed or measured. Thus, the methods of expressing
concentrations discussed so far are critical.
Writer
- Ajay Shende
- Subject Matter Expert (Chemistry)



Your idea about simplifying concentration of solution is very effective. Private tutor St. Augustine Thanks for sharing.
ReplyDeleteThanks for telling us the different methods Private tutor Ocala
ReplyDelete